
Training - Course WA
Context
Pediatric differentiated thyroid cancer (DTC) patients frequently present with lymph node and/or distant (lung) metastases. Such patients warrant an aggressive treatment consisting of surgical removal of all surgically accessible local metastases as well as further treatment with one or more courses of radioiodine therapy (RAI). It is still a subject of debate in literature how much I-131 should be administered to pediatric patients. Patients can either be given a fixed (possibly body weight adjusted) activity or a dosimetry based activity, which is often considerably higher.
Learning Objectives
Objective
Here, we will present a typical case of a pediatric patient who was treated using a dosimetric approach. Then we will discuss the basis of dosimetry and the procedures involved, followed by a discussion of when to use dosimetric RAI as well as the pros and cons of the various approaches in pediatric patients.
Results
In general, two opposite approaches to dosimetry exist: either the activity that is as high as safely administrable (AHASA) is determined based on the radiation exposure to the critical organs at risk (in pediatric patients these are the bone marrow and, in patients with lung metastases, the lungs), or a lesion-based approach in which the activity that is required to deliver a certain radiation dose to the metastatic lesion(s) is determined.
Conclusion
Because the latter approach requires an accurate volumetry of the target lesion(s), which is not possible in children with disseminated pulmonary metastases, which are often not visible with morphologic imaging techniques, we advocate using the AHASA approach in children with extensive metastatic DTC.
Frederik A. Verburg, Christoph Reiners, and Heribert Hänscheid
DOI: http://dx.doi.org/10.1210/jc.2013-2259
Received: May 16, 2013
Accepted: July 19, 2013
Published Online: December 04, 2013
Authors, editors, and Endocrine Society staff involved in planning this JCEM Journal-based CME activity are required to disclose to The Endocrine Society and to learners any relevant financial relationship(s) of the individual or spouse/partner that have occurred within the last 12 months with any commercial interest(s) whose products or services are discussed in the CME content. The Endocrine Society has reviewed all disclosures and resolved all identified conflicts of interest.
The following authors reported relevant financial relationships:
Frederik A. Verburg, M.D., Ph.D., has received speakers' fees and research support from Genzyme Corp. and is a paid member of an advisory board for Roche Healthcare. Christoph Reiners, M.D., has received speakers' fees and research support from Genzyme Corp.
The following author reported no relevant financial relationships:
Heribert Hänscheid, M.D., has no relevant financial relationships.
The following JCEM Editors reported relevant financial relationships:
The Editor-in-Chief, Leonard Wartofsky, M.D., is a Consultant for Asurogen, Genzyme, and IBSA, and is on the Speaker's Bureau for Genzyme.
Kenneth Burman, M.D., is a Consultant for Medscape and UpToDate; a Reviewer for the Endocrine Fellows Foundation; and has received Institutional Grants for Research from Amgen, Eisei, and Pfizer.
Lynnette Nieman, M.D., is an Author/Editor for UpToDate, and receives Research Support from HRA-Pharmaceutical.
The following JCEM Editors reported no relevant financial relationships: Paolo Beck-Peccoz, M.D.; David Ehrmann, M.D.; David Handelsman, Ph.D.; Michael Kleerekoper, M.D.; Merrily Poth, M.D.; Constantine Stratakis, M.D.
Endocrine Society staff associated with the development of content for this activity reported no relevant financial relationships.
Acknowledgement of Commercial Support
JCEM Journal-based CME activities are not supported by grants, other funds, or in-kind contributions from commercial supporters.
Required Hardware/software
I. Introduction
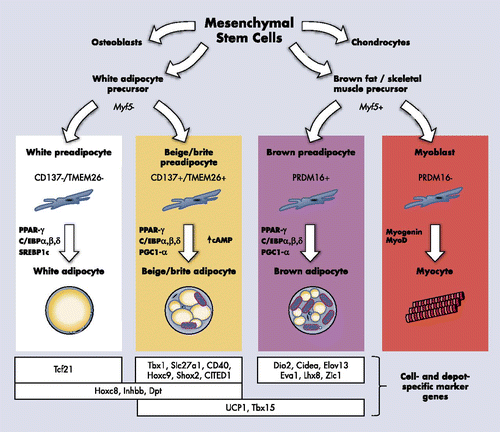
Adipose tissue plays a central role in the interplay between nutrition, energy balance, and human health. There are 2 types of adipose tissue, white and brown. White adipose tissue (WAT) stores energy, whereas brown adipose tissue (BAT) dissipates it. Overnutrition and/or physical inactivity result in an excess of WAT, the hallmark of obesity. In contrast, BAT is thermogenic, a property conferred by the presence of a unique protein, uncoupling protein 1 (UCP1). Located in the inner mitochondrial membrane, UCP1 uncouples mitochondrial respiration, releasing energy as heat. This unique property protects animals from hypothermia (1).
The traditional belief that BAT exists only in infants but not in adults has resulted in a paucity of research in humans. However, the discovery of fat with high metabolic activity in adults by functional imaging using positron emission tomography (PET) brought about a resurgence in research interest on BAT identity, abundance, prevalence, regulation, and significance in humans (2–9).
This review will cover 1) the characteristics and ontogeny of BAT, 2) its prevalence and regulation, 3) metabolic relevance, 4) the potential roles of BAT in health and diseases, and 5) the avenues for therapeutic targeting of BAT in obesity. These questions will be discussed on the background of known biology of BAT in rodents.

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward